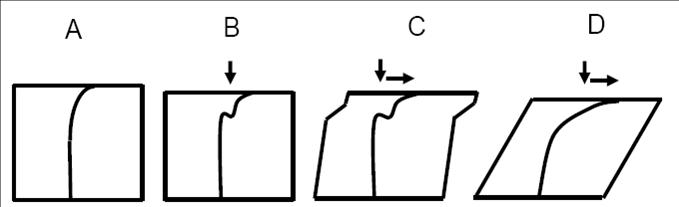
Articular cartilage, the soft connective tissue that coats bones in joints, is a highly complex and inhomogeneous material. It is made up of a fluid-saturated, cross-linked network of collagen fibrils whose orientation and porosity vary with depth from the articular surface. Interspersed among the network are cells and highly charged molecules called proteoglycans. Cell shape, cell density and proteoglycan density are all also spatially dependent.
In vivo, this tissue is constantly subject to both shear and axial forces. However, the depth dependence of its shear properties are poorly understood. Given the fact that cartilage damage due to osteoarthritis exhibits clear spatial variations, measuring the spatially dependent shear properties in healthy and diseased articular cartilage could aid our understanding of the origin of osteoarthritis and assist in the development of a sensitive diagnostic tool for this disease.
In collaboration with the Lawrence Bonassar group in Cornell's Biomedical Engineering Department, we study the spatially-dependent shear mechanical properties of this tissue using a novel confocal microscopy strain-mapping technique and a custom tissue shearing device developed in collaboration with Harrick Scientific.
This device allows us to track fluorescently stained cells or extracellular matrix components in sheared articular cartilage explants and use the resulting displacement profiles and shear force measurements to map the shear modulus as a function of depth. Using this methodology, we have discovered that under static loads, articular cartilage is weakest under shear at a distance of around 100 um from the articular surface. Here, collagen fibrils are more randomly oriented than fibrils at the surface or deeper in the tissue.
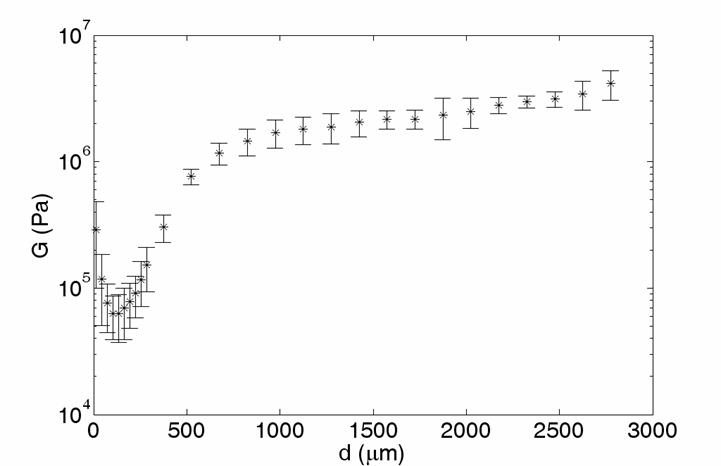
We have also found that the mechanical properties are particularly nonlinear in this region. Specifically, it becomes more difficult to shear at large shear strains, but easier to shear at large axial strains. On the other hand, increasing the axial strain makes this weak region more compliant to the the rest of the tissue.
Building on these observations, we have developed a simple thought model that describes the nonlinear behavior of articular cartilage in terms of localized buckling of collagen fibrils. We hypothesize that the effect of small shear strains is to bend buckled collagen fibrils in the weakest region into alignment. Since collagen is much easier to bend than to stretch, this region is initially the weakest area of tissue under shear. However, once the collagen fibrils in the weak region become aligned and taught, this region stiffens.